Which Change Of State Involves A Release Of Energy? Boiling Condensation Melting Sublimation
Chapter 10. Solids and Liquids
Phase Transitions: Melting, Boiling, and Subliming
- Describe what happens during a phase change.
- Summate the energy change needed for a stage change.
Substances tin can change phase — often because of a temperature modify. At low temperatures, nigh substances are solid; as the temperature increases, they become liquid; at higher temperatures nevertheless, they get gaseous.
The process of a solid condign a liquid is called melting (an older term that yous may see sometimes is fusion). The opposite process, a liquid becoming a solid, is chosen solidification. For any pure substance, the temperature at which melting occurs — known every bit the melting point — is a characteristic of that substance. It requires free energy for a solid to cook into a liquid. Every pure substance has a certain amount of energy information technology needs to change from a solid to a liquid. This amount is chosen the enthalpy of fusion (or heat of fusion) of the substance, represented as ΔH fus. Some ΔH fus values are listed in Table x.2 "Enthalpies of Fusion for Diverse Substances"; it is assumed that these values are for the melting signal of the substance. Note that the unit of measurement of ΔH fus is kilojoules per mole, and then we demand to know the quantity of fabric to know how much energy is involved. The ΔH fus is always tabulated as a positive number. However, it tin be used for both the melting and the solidification processes as long as you go on in listen that melting is always endothermic (so ΔH will exist positive), while solidification is e'er exothermic (and so ΔH will be negative).
| Substance (Melting Bespeak) | ΔH fus (kJ/mol) |
|---|---|
| Water (0°C) | 6.01 |
| Aluminum (660°C) | 10.7 |
| Benzene (v.5°C) | 9.95 |
| Ethanol (−114.three°C) | five.02 |
| Mercury (−38.8°C) | 2.29 |
What is the energy alter when 45.7 one thousand of H2O melt at 0°C?
Solution
The ΔH fus of HiiO is vi.01 kJ/mol. However, our quantity is given in units of grams, not moles, then the outset step is to convert grams to moles using the molar mass of HtwoO, which is 18.0 g/mol. And then we tin use ΔH fus as a conversion factor. Because the substance is melting, the process is endothermic, so the energy change volition accept a positive sign.
![]()
Without a sign, the number is causeless to be positive.
Examination Yourself
What is the energy change when 108 g of CsixHsix freeze at 5.5°C?
Answer
−thirteen.8 kJ
During melting, energy goes exclusively to irresolute the phase of a substance; it does not become into irresolute the temperature of a substance. Hence melting is an isothermal process considering a substance stays at the aforementioned temperature. Only when all of a substance is melted does whatever additional energy become to changing its temperature.
What happens when a solid becomes a liquid? In a solid, individual particles are stuck in place considering the intermolecular forces cannot be overcome by the free energy of the particles. When more energy is supplied (east.g., by raising the temperature), in that location comes a point at which the particles have plenty free energy to move around but non enough energy to split up. This is the liquid phase: particles are yet in contact but are able to move around each other. This explains why liquids can assume the shape of their containers: the particles move around and, under the influence of gravity, make full the lowest volume possible (unless the liquid is in a zero-gravity environment — see Figure 10.sixteen "Liquids and Gravity").
The phase change between a liquid and a gas has some similarities to the phase change between a solid and a liquid. At a sure temperature, the particles in a liquid have enough free energy to become a gas. The process of a liquid becoming a gas is called boiling (or vapourization), while the process of a gas condign a liquid is called condensation. Even so, different the solid/liquid conversion process, the liquid/gas conversion process is noticeably affected past the surrounding pressure on the liquid because gases are strongly affected by pressure. This ways that the temperature at which a liquid becomes a gas, the humid point, tin can change with surrounding pressure. Therefore, we define the normal boiling point as the temperature at which a liquid changes to a gas when the surrounding pressure level is exactly one atm, or 760 torr. Unless otherwise specified, it is assumed that a boiling signal is for 1 atm of pressure.
Like the solid/liquid phase alter, the liquid/gas phase change involves energy. The amount of energy required to convert a liquid to a gas is called the enthalpy of vaporization (or heat of vaporization), represented as ΔH vap. Some ΔH vap values are listed in Tabular array 10.3 "Enthalpies of Vaporization for Various Substances"; it is assumed that these values are for the normal humid point temperature of the substance, which is too given in the tabular array. The unit for ΔH vap is also kilojoules per mole, so nosotros need to know the quantity of material to know how much free energy is involved. The ΔH vap is also always tabulated every bit a positive number. It tin be used for both the humid and the condensation processes equally long as yous go along in listen that boiling is always endothermic (so ΔH will be positive), while condensation is always exothermic (and then ΔH volition be negative).
| Substance (Normal Boiling Indicate) | ΔH vap (kJ/mol) |
|---|---|
| Water (100°C) | 40.68 |
| Bromine (59.five°C) | 15.4 |
| Benzene (80.1°C) | 30.8 |
| Ethanol (78.iii°C) | 38.six |
| Mercury (357°C) | 59.23 |
What is the free energy alter when 66.7 m of Br2(k) condense to a liquid at 59.5°C?
Solution
The ΔH vap of Br2 is 15.4 kJ/mol. Fifty-fifty though this is a condensation process, we can however apply the numerical value of ΔH vap every bit long every bit we realize that we must take energy out, then the ΔH value volition exist negative. To decide the magnitude of the free energy modify, we must get-go convert the amount of Br2 to moles. And then nosotros can use ΔH vap as a conversion factor.
![]()
Considering the process is exothermic, the actual value will exist negative: ΔH = −6.43 kJ.
Test Yourself
What is the free energy change when 822 g of CtwoH5OH(ℓ) boil at its normal boiling point of 78.3°C?
Reply
689 kJ
As with melting, the free energy in boiling goes exclusively to changing the stage of a substance; it does not go into changing the temperature of a substance. Then boiling is also an isothermal procedure. Only when all of a substance has boiled does any boosted energy go to changing its temperature.
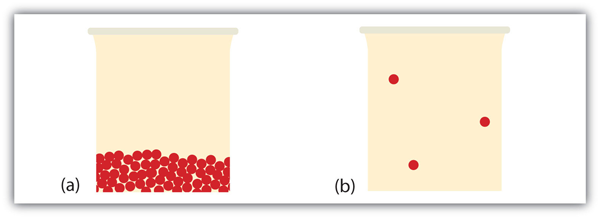
What happens when a liquid becomes a gas? We have already established that a liquid is composed of particles in contact with each other. When a liquid becomes a gas, the particles carve up from each other, with each particle going its own manner in space. This is how gases tend to fill their containers. Indeed, in the gas phase most of the volume is empty infinite; only about one ane-thousandth of the volume is actually taken up by thing (run into Effigy 10.17 "Liquids and Gases"). It is this belongings of gases that explains why they can be compressed, a fact that is considered in Chapter half-dozen "Gases".
Under some circumstances, the solid stage can transition directly to the gas stage without going through a liquid phase, and a gas can directly become a solid. The solid-to-gas change is called sublimation, while the reverse process is called deposition. Sublimation is isothermal, like the other phase changes. There is a measurable energy change during sublimation; this free energy modify is called the enthalpy of sublimation, represented as ΔH sub. The relationship between the ΔH sub and the other enthalpy changes is every bit follows:
ΔH sub = ΔH fus + ΔH vap
As such, ΔH sub is not always tabulated considering information technology can be only calculated from ΔH fus and ΔH vap.
There are several common examples of sublimation. A well-known production — dry ice — is really solid COtwo. Dry ice is dry considering it sublimes, with the solid bypassing the liquid phase and going direct to the gas phase. The sublimation occurs at temperature of −77°C, so information technology must be handled with caution. If yous accept ever noticed that ice cubes in a freezer tend to get smaller over time, it is because the solid h2o is very slowly subliming. "Freezer burn" isn't actually a burn; it occurs when certain foods, such as meats, slowly lose solid water content because of sublimation. The food is nonetheless good only looks unappetizing. Reducing the temperature of a freezer will dull the sublimation of solid water.
Chemical equations tin can be used to represent a phase alter. In such cases, it is crucial to utilize phase labels on the substances. For instance, the chemical equation for the melting of ice to make liquid water is equally follows:
H2O(s) → HtwoO(ℓ)
No chemical alter is taking place; still, a physical alter is taking place.
Heating Curves
A plot of the temperature versus the amount of heat added is known as a heating curve (see Figure 10.eighteen). These are normally used to visually show the human relationship between phase changes and enthalpy for a given substance.
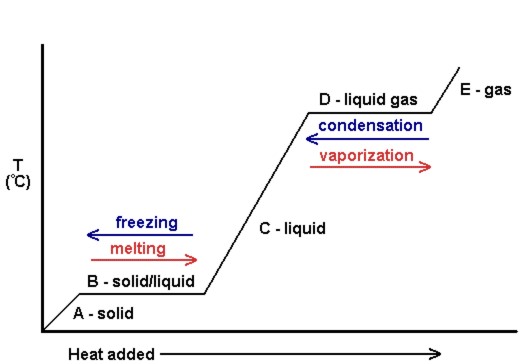
In Effigy 10.xviii[1], the solid gains kinetic free energy and consequently rises in temperature every bit heat is added. At the melting point, the rut added is used to intermission the attractive intermolecular forces of the solid instead of increasing kinetic energy, and therefore the temperature remains constant. Later on all the solid has melted, in one case over again, the oestrus added goes to increasing the kinetic energy (and temperature) of the liquid molecules until the boiling point. At the boiling indicate, once once more, the heat added is used to interruption the attractive intermolecular forces instead of supplying kinetic free energy, and the temperature remains constant until all liquid has been turned to gas.
- Phase changes tin can occur between any two phases of matter.
- All phase changes occur with a simultaneous change in energy.
- All phase changes are isothermal.
Questions
- What is the deviation between melting and solidification?
- What is the difference between humid and condensation?
- Describe the molecular changes when a solid becomes a liquid.
- Depict the molecular changes when a liquid becomes a gas.
- What is the energy modify when 78.0 thou of Hg melt at −38.viii°C?
- What is the energy modify when 30.8 g of Al solidify at 660°C?
- What is the energy alter when 111 g of Br2 boil at 59.v°C?
- What is the energy change when 98.half dozen g of H2O condense at 100°C?
- Each of the following statements is incorrect. Rewrite them so they are correct.
- Temperature changes during a phase change.
- The process of a liquid becoming a gas is called sublimation.
- Each of the following statements is wrong. Rewrite them so they are correct.
- The volume of a gas contains only virtually 10% thing, with the rest beingness empty infinite.
- ΔH sub is equal to ΔH vap.
- Write the chemical equation for the melting of elemental sodium.
- Write the chemical equation for the solidification of benzene (CsixH6).
- Write the chemical equation for the sublimation of COtwo.
- Write the chemical equation for the boiling of propanol (C3H7OH).
- What is the ΔH sub of H2O? (Hint: see Tabular array x.2 "Enthalpies of Fusion for Diverse Substances" and Table 10.3 "Enthalpies of Vaporization for Diverse Substances".)
- The ΔH sub of I2 is 60.46 kJ/mol, while its ΔH vap is 41.71 kJ/mol. What is the ΔH fus of I2?
Answers
- Melting is the stage alter from a solid to a liquid, whereas solidification is the phase change from a liquid to a solid.
- The molecules accept enough energy to move about each other only not enough to completely separate from each other.
- 890 J
- 10.7 kJ
-
- Temperature does not change during a phase alter.
- The process of a liquid condign a gas is called boiling; the procedure of a solid becoming a gas is called sublimation.
- Na(s) → Na(ℓ)
- CO2(s) → CO2(g)
- 46.69 kJ/mol
Media Attributions
- "Drinking glass of Water" © 2005 by Derek Jensen is licensed nether a Public Domain license
- "Clayton Anderson zero one thousand" © 2010 by NASA is licensed under a Public Domain license
Source: https://opentextbc.ca/introductorychemistry/chapter/phase-transitions-melting-boiling-and-subliming/
Posted by: martinpervou.blogspot.com


0 Response to "Which Change Of State Involves A Release Of Energy? Boiling Condensation Melting Sublimation"
Post a Comment